In the race to develop effective treatments for patients infected with COVID-19, researchers are employing an innovative study design to evaluate the clinical efficacy, as assessed by time to recovery, of multiple different investigational therapeutics. The study, sponsored in the U.S. by the National Institutes of Allergy and Infectious Disease, is a multicenter trial being conducted at approximately 100 sites globally, with participation from 41 U.S. based and 19 international health systems, university medical centers and national research centers.1
Gold Standard Approach
The two-arm Randomized Controlled Trial (RCT) is the gold standard approach for evaluating new drugs wherein study participants are assigned to one of the two trial arms, the first group receiving the experimental treatment while the second group is given a placebo or the standard existing treatment. In response to the COVID-19 pandemic, and attempting to evaluate the large number of new and existing treatments, researchers recognized the limitations of the two-arm RCT model; specifically that by assigning half of the participants to a placebo or less effective treatment, many could be denied their best chance at recovery, and that setting up a trial to evaluate each treatment independently might take years of research and require the enrolling thousands of study participants.
Adaptive Clinical Trial Approach
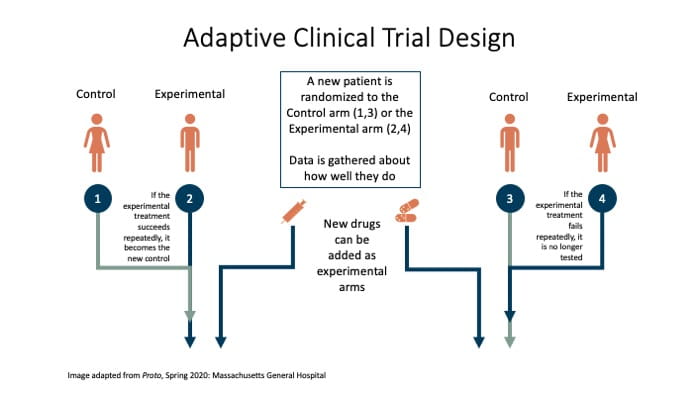
In an adaptive trial, study participants are randomly and blindly assigned to either a control arm or an experimental treatment arm; more than one experimental treatment may be included at the outset of the trial. Additional arms and protocols may then be added or removed from a trial depending on early evaluation of the drugs’ effects. For example, if one treatment regimen seems to be more successful, researchers can increase the number of participants receiving that treatment. Similarly, ineffective treatments can be removed early, and new treatments can be initiated quickly, enabling patients to receive the most promising treatments faster.2 This may be especially important in the context of an infectious pandemic, where symptoms and disease progression are not well understood. With over two million reported COVID-19 cases in the U.S. and growing, time is of the essence.
Possible Benefits of Adaptive Clinical Trial Model for COVID-19
The design of an adaptive clinical trial offers faster detection of benefits or harms associated with experimental therapies, drugs that are showing benefits can be moved to the study’s control arm and drugs that aren’t helping patients can be removed from the trial. Additionally, as researchers are able to use the same control group to measure the effectiveness of experimental treatments, fewer trial participants need to be enrolled. The adaptive design means promising therapies which demonstrate efficacy early on could become the standard of care for patients later in the trial.
The Adaptive COVID-19 Treatment Trial will initially study the drug Remdesivir (experimental) against placebo (control) and will look to introduce new therapeutic treatments over time. The study design ensures interim monitoring to introduce new arms and allow early stopping for futility, efficacy, or safety. To control for background standards of supportive care, which may evolve or improve over time as more is learned about successful management of COVID-19, comparisons of safety and efficacy will be based on data from concurrently randomized subjects.
A preliminary report of findings from one of the study sites concluded “Remdesivir was superior to placebo in shortening the time to recovery in adults hospitalized with Covid-19 and evidence of lower respiratory tract infection.”3
With millions of lives potentially impacted by this timely and important treatment research, it is encouraging to see the unique and flexible elements of this model put to good use.
1https://clinicaltrials.gov/ct2/show/NCT04280705
2https://nfcr.org/blog/understanding-adaptive-clinical-trials/?gclid=EAIaIQobChMI09L5zdSY6gIVD4zICh0a-Q1REAAYASAAEgKyRfD_BwE
3https://www.nejm.org/doi/full/10.1056/NEJMoa2007764
Meet NextGen Ambient Assist, your new AI ally that generates a structured SOAP note in seconds from listening to the natural patient/provider conversation.
Read Now